How can I draw 1,2,3,4,5,6-hexachlorocyclohexane with all the chloro groups in axial positions?
1 Answer
There are two kinds of substituents that can be attached to a chair conformation for a cyclohexane - axial substituents and equatorial substituents.
The axial substituents are drawn pointing straight up and straight down on the chair conformation, while the equatorial substituents are drawn pointing out to the sides of the chair.
For cyclohexane, or
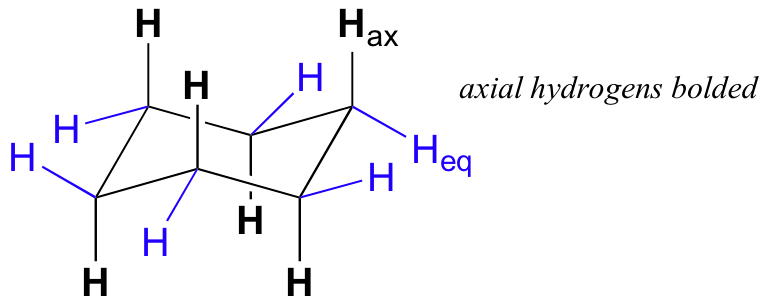
As you can see, the axial hydrogens alternate between UP and DOWN positions on the chair.
UP is a term used to describe groups that are coming out of the plane of the page (are placed on a wedge), while DOWN is used to describe groups that are going into the plane of the page (are placed on a dash).
For hexachlorocyclohexane, or
If you start at the carbon labeled (1) and go clockwise through all the axial chloro groups, youl have UP, DOWN, UP, DOWN, UP, and finally DOWN (the chlorine atom attached to the carbon labeled (6)).
In the same order, the chlorine atoms will be placed on a wedge (carbon 1), on a dash, on a wedge, on a dash, on a wedge, and finally on a dash (carbon 6). The wedge-dash notation for 1,2,3,4,5,6-hexachlorocyclohexane with all the chloro substituents in axial positions looks like this

