How can I draw the structures of all the geometric isomers of #[Ru(H_2O)_2(NH_3)_2Cl_2]^+# . What are the mirror images of any of these chiral molecules?
1 Answer
Jul 10, 2015
Explanation:
Let
There are five isomers:
(1) trans-
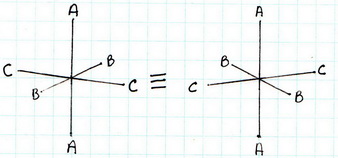
(2) trans-
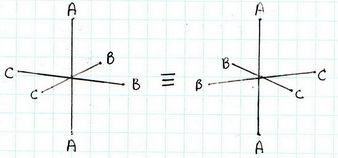
(3) cis-
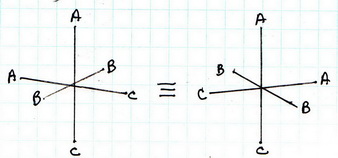
(4) cis-
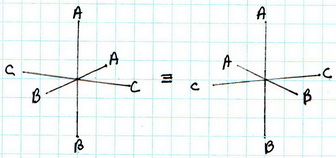
(5) cis-
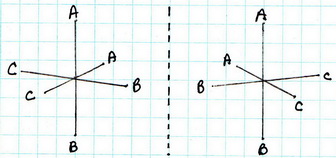
The all-cis isomer is not superimposable on its mirror image. It exists as a pair of enantiomers.

