How can I make a sodium atom model?
1 Answer
We draw atomic structures for any element with the help of atomic number they have. So, if you want to draw atomic structure for sodium first of all you should know the atomic number for sodium.
Atomic number of sodium is 11. Atomic number of any element refers to the number of electrons in an atom of that element they are having.
STEP 1. Always write the atomic number of the element first. Once you know its atomic number you know the number of protons and electrons in it. As in every stable atom the number of protons is equal to the number of electrons. Therefore, now you know the number of electrons too.
STEP 2. Do the electronic configuration for the atom of the element. We always do electronic configuration for the number of electrons present in the atom of the element. The number of elecrons each shell of an element can old is given by
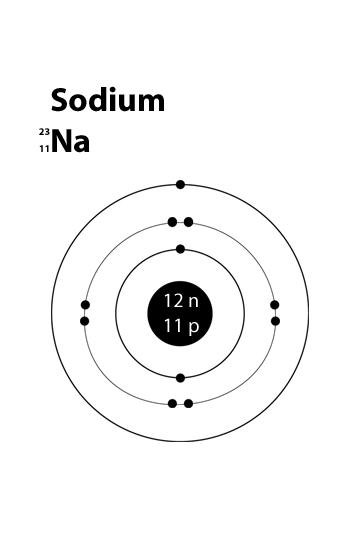
STRUCTURE FOR SODIUM
Atomic Number:- 11
Number of protons:- 11
Number of electrons:- 11
Electronic configuration:- K - 2, L-8, M-1
How do we know how many eletrons should be added to the first shell?
How many electrons should be added in the shell is given by
So, for counting the number of lectrons in the first shell we again apply the rule as:-
Here we multiplied 1 with 2 because we are calculating the number of electrons in the first shell.So, you can calculate the number of electrons for upcoming shells too.

