How can I report #""^13"C"# NMR data?
2 Answers
In a typical lab report, I would expect someone to report the following data:
- Number of unique carbons in the molecular formula
- Number of distinct
#""^13 C# peaks seen in the spectrum - Presence or absence of symmetry (such as in a benzene ring with two identical substituents para or meta to each other)
- Chemical shifts of each unique carbon in
#"ppm"# - Proposed carbon types (e.g.
#"C sp"^3# (likely alkyl) bonded to carbonyl#"C sp"^2# ); this can be an educated guess - Your carbon labels (e.g.
#"C"_A, "C"_B# , etc.)
This is how I tended to do it when I took O-Chem:
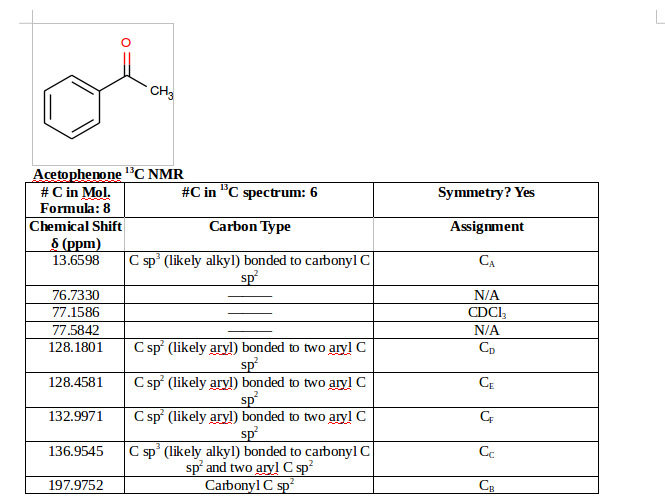
Based on the table, I said that
In published research papers though, you should not have any row borders (except those below a heading), nor outermost column borders, at least by ACS convention. Also, you would need a table caption at the top, explaining briefly what the table is showing.
For a lab report, you follow the format specified by your instructor.
Explanation:
If your instructor has not specified a format, Truong-Son N. has provided you with an excellent template in the preceding answer.
Research journals usually have their own formats for the reporting of
These include rules such as:
- The peak shifts should be rounded off to the nearest 0.1 ppm except when greater precision is needed to distinguish closely spaced peaks.
- Information about numbers of hydrogen atoms may be included, in order of increasing number of attached hydrogens.
- Detailed peak assignments should not be included in the Experimental section (they go in the Discussion section).
The usual format is:
Thus, you might see spectra reported in formats such as:



