How can you distinguish between cis and trans isomers?
1 Answer
cis and trans isomers are geometric isomers, which differ from each other in stability due to the relationship of each major substituent considered with respect to the other.
These can be represented in a few ways.
Essentially how you tell the difference is by knowing that cis means "same side" and trans means "opposite side".
CIS/TRANS ALKENES
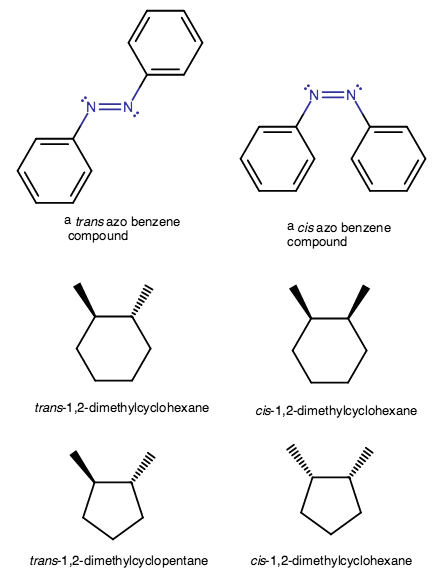
Take the trans azo benzene compound for example. It is an alkene, and in alkenes you may see cis and trans isomers.
It is almost completely rigid, so it cannot rotate its
You can see that the trans isomer's phenyl rings, which are "major substituents", are across and thus farther from each other, so it is a trans compound.
On the other hand, the cis isomer has more steric clash because the phenyl rings are on the same side and thus closer together, so the trans isomer is more stable.
CIS/TRANS RINGS
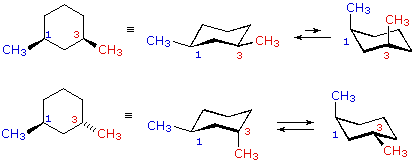
When we examine the cyclohexanes and cyclopentanes at the bottom, we see that trans isomers have two substituents on the ring that are related to each other. Each is either facing towards you (wedge) or away from you (dashed wedge).
When they aren't facing the same way, they are trans, and when they are, they are cis.
This is because their actual structures are not planar. Cyclohexanes prefer the chair conformation, while cyclopentanes are more like envelopes.