How can you tell if an element wants to gain or lose electrons?
1 Answer
Aug 1, 2017
Metals lose electrons, nonmetals gain electrons
Explanation:
In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Hydrogen is an exception, as it will usually lose its electron. Metalloids and some metals can be can lose or gain electrons.CK-12 Foundation
This is not always true, as elements such as nitrogen can lose electrons to become positive. When an ionic compound forms, the more electronegative element will gain electrons and the less electronegative element will lose electrons. 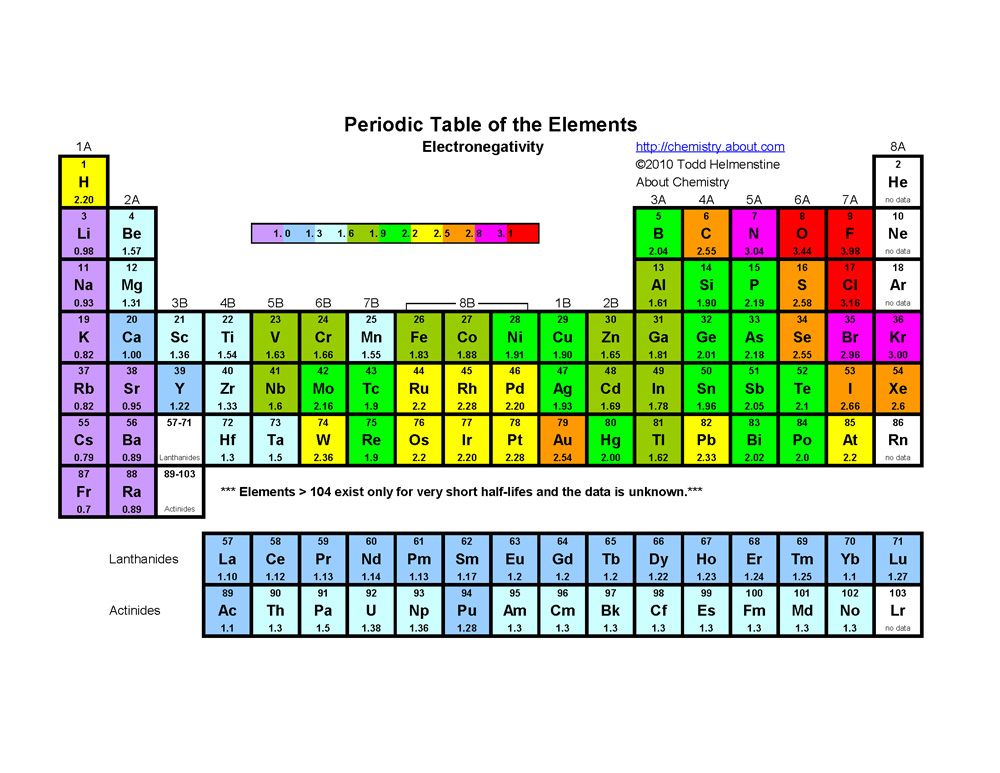 ThoughtCo
ThoughtCo

