How can you tell in a molecule if the atom is electronegative or not?
1 Answer
Jan 6, 2016
Every atom in a molecule has an electronegativity.
Explanation:
However, some atoms are more electronegative than others.
What's important in a molecule is the electronegativity difference between the two atoms of a bond.
You get the qualitative information from electronegativity trends in the Periodic Table.
Some Periodic Tables list the electronegativity values.
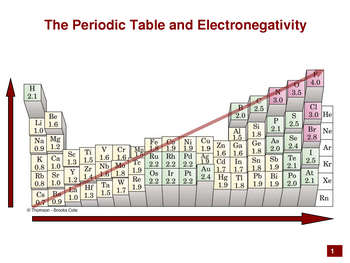
(from www.drcruzan.com)
Electronegativity increases from left to right and from bottom to top of the Periodic Table.
Elements that are further apart from each other have the greater electronegativity difference.

