How do anhydrides react with water?
1 Answer
At the carbonyl carbon that belongs to the bigger half.
A general anhydride looks like this:
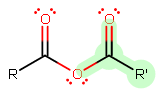
The leaving group is highlighted, assuming
A general rule of thumb is that the longer the alkyl chain of the
Since we are assuming that
An anhydride literally lacks water. (anhydrous = without water.)
Let's add water.
- Water attacks the carbonyl carbon. Which one? Pick the one that makes the carboxylate with the
#R'# leave, because that's what we're assuming will happen. This is reversible, and actually is slightly favored in the reverse direction, so we should add quite a bit of water to push the equilibrium forward. - Proton transfer via a suitable base. Naturally, the water we added. But this is still an equilibrium.
- Something has to leave to stabilize this tetrahedral intermediate. We don't want the water we just added to gain a proton and then leave. That's the reverse reaction. And of course,
#"O"^(2-)# is a much stronger base than#"R"'"OO"^(-)# simply by looking at its charge. So, the#\mathbf(R"'")# carboxylate group must leave. At this point it's irreversible. - The carboxylate would want a proton. Why? The pKa of a typical carboxylic acid is about
#5# , but the pKa of hydronium is about#-1.7# . The weaker acid exists in its acidic form predominantly. Hence, hydronium gives up its proton nicely.
You can now see that two carboxylic acids form.
CHALLENGE: Can you draw the mechanism for the dehydration of two carboxylic acids into one anhydride? Do you think it requires heat? How about acid?

