The key word is #"connectivity"#. What do I mean? If we take a simple molecule, butene, #"1-butene"# and #"2-butene"# are structural isomers in that they have the SAME chemical formula, #C_4H_8#, but clearly #H_2C=CHCH_2CH_2# is different to #H_3C-CH=CH-CH_2# given the position of the olefinic bond.
Now, we keep #"2-butene"# as our examplar, and consider the phenomenon of geometric isomerism. #"2-butene"# can generate 2 geometric isomers, #"cis"# or #"trans"# depending on the orientation of the pendant methyl groups: are they on the same side of the double bond, #"cis"#, or on opposite sides of the double bonds, #"trans"#?
See here
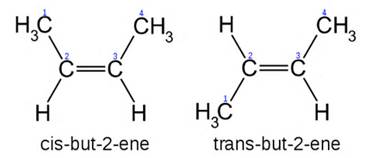
Note that the connectivity of each molecule is identical, #C1# connects to #C2#.......to #C3#, yet their geometry is manifestly different.
This different geometry confers different chemistry, and different physical properties. The cis isomer, for instance, has a melting point of #-138.9# #""^@C# versus a melting point of #-105.5# #""^@C# for the trans isomer. This difference in physical properties, and certainly other differences could be quoted with respect to chemical reactivity as well, illustrates the importance of structure.
Handedness is another manifestation of geometric isomerism, but we'll leave that for another session.


