How do geometric isomers occur?
1 Answer
May 15, 2017
See below.
Explanation:
Geometric isomerism comes from the fact that compounds containing double bonds show restriction of rotation about the double bond (C=C).
So in order for a compound to exhibit geometric isomerism it must have:
1. a double bond i.e. an alkene linkage.
2. two identical groups attached to the adjacent C atoms
3. two different groups attached to each C on the double bond.
Example: but-2-ene
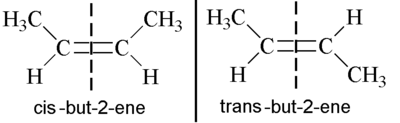 wikibooks
wikibooks
looking at the isomers of but-2-ene, it has one identical group attached on each side of the double bond (H atoms) and also a methyl group ( a different group from the H atoms) attached on each side of the double bond.
Hope this wasn't too confusing :)

