How do I determine the bond angle in a molecule?
1 Answer
There are three basic steps to determining the bond angles in a molecule:
Explanation:
1. Write the Lewis dot structure for the molecule.
Assume that you must determine the bond angles in
If we have three
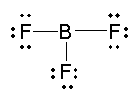
This gives us three bonding pairs of electrons and 0 nonbonding pairs.
Thus, the steric number, SN — the number of non-bonding and bonding electron groups (Note: single, double, and triple bonds all count as one electron group).
The SN is also known as the number of ELECTRON DOMAINS.
2. Use the steric number and VSEPR theory to determine the electron domain geometry of the molecule.
To get the VSEPR geometry, imagine that there is a sphere around the central atom.
Place the electron pairs on the surface of the sphere so that they are as far apart as possible.
This is how two to six electron domains arrange themselves on the surface of a sphere.
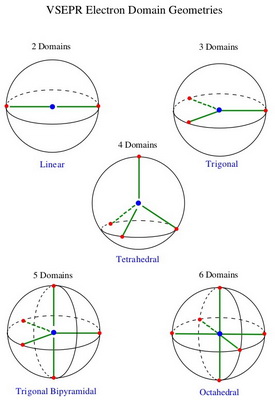
Three electron groups arrange themselves evenly around the equator of the sphere to give a trigonal planar shape.
3. Use the VSEPR shape to determine the angles between the electron domains.
From elementary math, we know that a circle is composed of 360 °.
We divide this number by the number of electron domains and get
Thus, the bond angles in
This website will be a useful help in understanding how the above method works.


