How do I use Lewis structures to determine the oxidation numbers of #"N"# in compounds like #"N"_2"O", "NO", "N"_2"O"_3, "N"_2"O"_4#, and #"N"_2"O"_5#?
1 Answer
Warning! Long answer. You count the valence electrons around
Explanation:
The Rules
- Lone pair electrons (LPs) belong entirely to the atom on which they reside.
- Shared electrons (bonding pair electrons or BEs) between identical atoms are shared equally.
- Shared electrons between different atoms belong entirely to the more electronegative atom.
- Oxidation number (ON) is the difference between the valence electrons in the isolated atom (VE) and the valence electrons in the bound atom (LP + BE).
#color(blue)(bar(ul(|color(white)(a/a) ON = VE - LP - BE color(white)(a/a)|)))" "#
I will calculate the oxidation numbers for only one Lewis structure of each oxide.
An isolated
Dinitrogen monoxide
The Lewis structure of
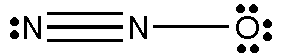
(Adapted from Chegg)
(a) The left hand
(b) The central
Each
The average oxidation number on
Nitrogen monoxide
The Lewis structure of

(Adapted from PEOI)
Dinitrogen trioxide
The Lewis structure of
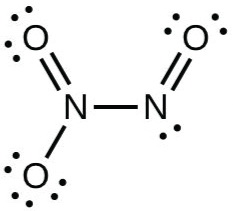
(Adapted from Chegg)
(a) The left hand
(b) The central
Each
The average formal charge on
Dinitrogen Tetroxide
The Lewis structure of
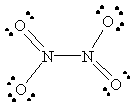
(Adapted from archives.evergreen.edu)
(a) The left-hand
(b) The right-hand
Each
The average oxidation number on
Dinitrogen pentoxide
The Lewis structure of
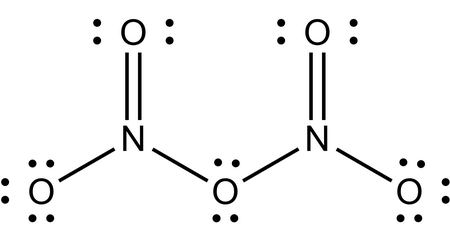
(a) The left-hand
(b) The right-hand
Each
The average oxidation number on

