How do minerals change without melting?
1 Answer
Pseudomorphism!
Explanation:
Cool word isn't it?
This is a really cool way that a mineral can change its atomic structure without melting. But note that the mineral doesn't change its chemical composition in this case.
An example, a mineral such as Kyanite (
At low pressure (a few kilobars, roughly a few kilometres into earths crust) andalusite is the stable structure and then at higher temperature moderate pressure sillimanite is the stable structure.
So as we change pressure and temperature a different atomic structure becomes stable and the mineral changes.
Another example is diamond. Diamond is simply carbon (
Though, I wouldn't recommend putting your pencil in the oven for a few weeks to try and make diamond! But expose diamond to some high temperature and pressure (below its stability field) and it might just transform back to graphite!
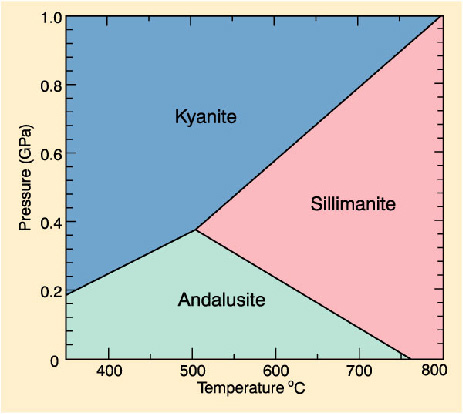
Above is the stability ranges for the three guys I mentioned above, diamond occurs four times higher than this diagram (more than 120km into our mantle)!
NB: