How do the three states of matter change?
1 Answer
Jul 31, 2017
Matter changes phases via the addition or removal of heat.
- process:
#\color(navy)(solid)\color(indianred)(\rarr+heat)\rArr\color(steelblue)(liquid)color(indianred)(\rarr+heat)\rArr\color(royalblue)(gas)#
and vice versa

Explanation:
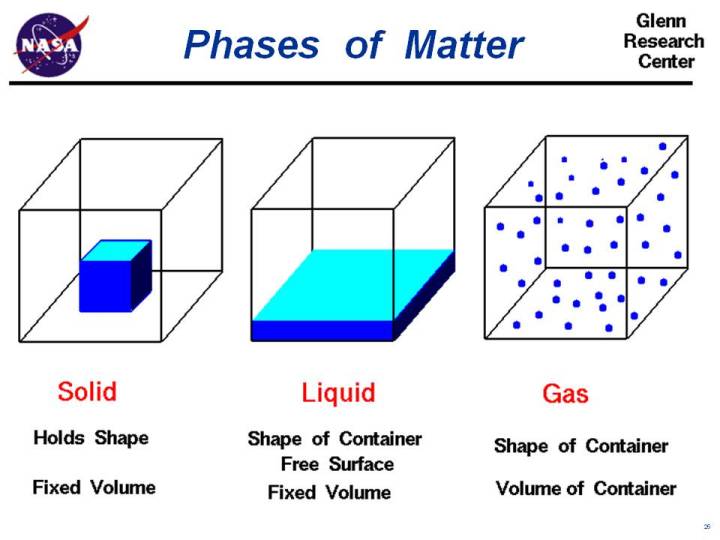
Matter changes phases (states) by either addition or removal of heat. For example, adding heat to ice causes it to melt. This creates water, a liquid. If you add even more heat, then the water will evaporate and become vapor, a gas.
Conversely, if you remove heat from gas, it will reverse back to a liquid (i.e. rainfall occurs in low temperatures); removing more will cause the liquid to freeze into a solid (i.e. ice on a winter day).
There are cases where a substance will skip the liquid phase and transition directly to gas when heat is added; this is called sublimation. Reversing the process is called deposition/desublimation.

