How do valence electrons relate to the periodic table?
1 Answer
Valence electrons relate to the position of elements within the groups and periods of the periodic table, and also their position within blocks.
Explanation:
The number of valence electrons an atom has determines its location in the period: the element with the electron configuration of
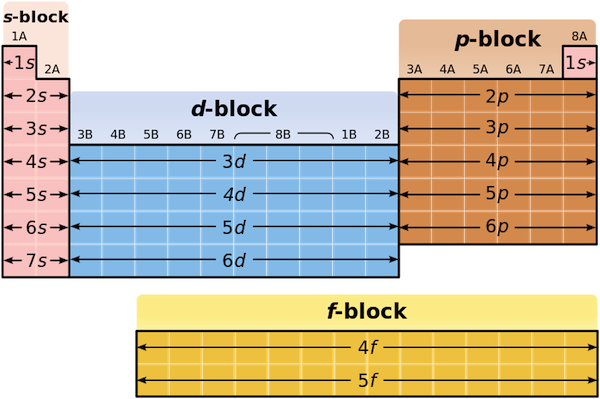
The periodic table can also be divided up into blocks, depending on the highest orbital of an atom of that element's electron configuration. Once more, sulfur's electron configuration is

