How do we consider that a p orbital in benzene consists of 6 electrons?
1 Answer
Apr 20, 2018
It does not! No
Each
The SIX
Here is another depiction of that delocalization.
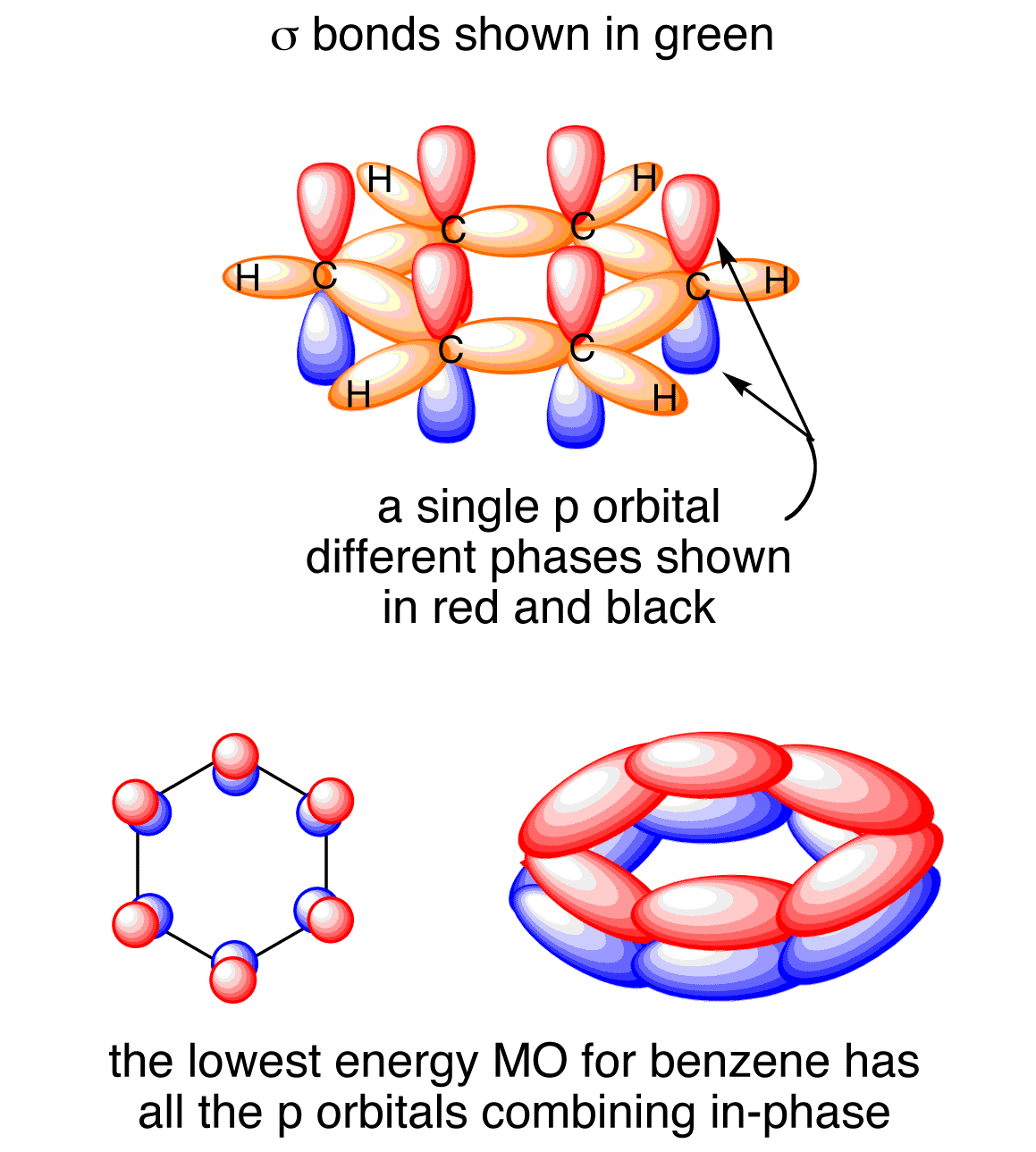
It does not! No
Each
The SIX
Here is another depiction of that delocalization.
