How do you alkylate an aldehyde?
1 Answer
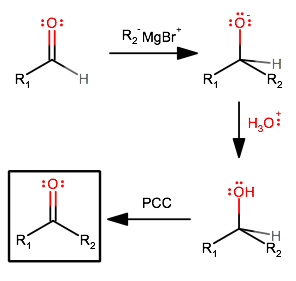
If you really think about it, what you want is to add an alkyl group. What that means is you need to couple a compound that happens to be an alkyl-based compound with an electrophile (aldehyde carbonyl carbon). That means you need a nucleophile that is alkyl-based. The one you probably learned in class is a Grignard reagent. i.e.:
#"R"-"MgBr"#
I assume you mean simply adding an alkyl group on. However, it's not quite that simple. You'd get an alkoxide intermediate on which there are no available leaving groups that can leave.
That means you'd have to oxidize the resultant secondary alcohol.
We can use something like
And the overall synthesis would look like this: