How do you alkylate benzene?
1 Answer
Dec 1, 2015
You can do it via a Friedel-Crafts Alkylation, where you have an alkyl halide and a strong Lewis acid catalyst. Commonly we use
The mechanism is as follows, and is very similar to Friedel-Crafts Acylation (and even draws parallels with the benzene halogenation reaction where you have
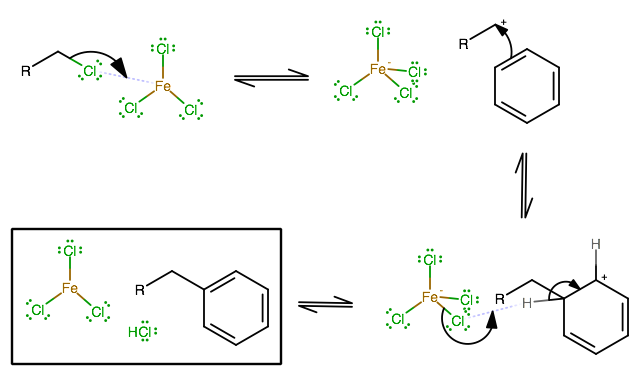
It is worth noting though that by adding an alkyl group onto benzene, you've added an activating group; that is, the alkyl group donates electrons to the ring, making it more nucleophilic, and thus more prone to reacting in this Friedel-Crafts Alkylation.
As a result, you have a pretty good chance for overalkylation if you don't add a controlled amount of catalyst and reactant.

