How do you determine if you have an electrophile or a nucleophile?
1 Answer
May 31, 2016
It's not always a black/white "it's definitely this or that", but...
PROPERTIES OF TYPICAL NUCLEOPHILE
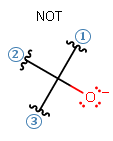
- Should be relatively sterically unhindered for nucleophilic behavior.
Example:
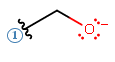
- If negatively-charged, consider the possibility of either basic behavior or nucleophilic behavior.
Example:
- If it has a lone pair of electrons, consider the possibility of either basic behavior or nucleophilic behavior. If so, then it should definitely be in an aprotic solvent.
Example:
#stackrel(..)("N")"H"_3# #"R"stackrel(..)("N")"H"_2# - etc.
- Should be in an aprotic solvent, especially if the nucleophile is negatively-charged or has a lone pair (could undesirably steal a proton and deactivate itself):
Example:
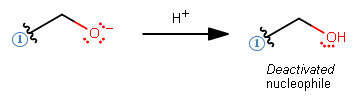
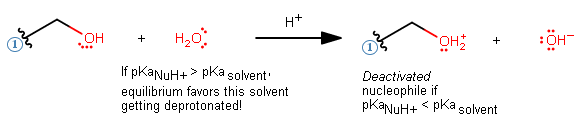
AND has a conjugate acid
#"pKa"# higher than the#"pKa"# of the solvent (remember the equilibrium lies on the side of the weaker acid, so the higher conjugate acid#"pKa"# means the equilibrium would favor protonating the nucleophile and deactivating it):Example:
PROPERTIES OF TYPICAL ELECTROPHILE
- If positively-charged, generally it makes a nice electrophile.
Example:
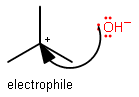
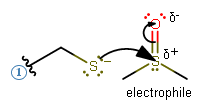
- If a particular atom would possess a partial positive formal charge in a fairly polar bond:
Examples:
#"HCl"# #"HBr"# #"HI"# - etc.
or if the molecule is polarizable so that the electron distribution can be disturbed towards the more electronegative atom (away from the nucleophile):
Examples:
#"HCl"# #"HBr"# #"HI"# #"Br"_2# #"I"_2# - etc.
then it is probably an electrophile.

