How do you draw ( S )-1-bromo-1-chlorobutane?
1 Answer
Here's how I do it.
Explanation:
Step 1. Draw the structure of 1-bromo-1-chlorobutane.
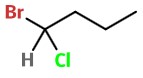
Step 2. To get the stereochemical structure, start with the structure of butane.
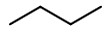
Step 3. At
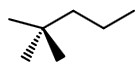
Step 4. Put the
We have a 50:50 chance of getting it right. Here's one possibility.
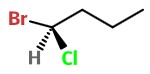
Step 5. Assign priorities to the groups.
Step 6. Assign stereochemistry
Then
We got it right!
So this really is (
If we had gotten it wrong, we would have interchanged any two of the groups.
For example, in the diagram above, interchanging the

