Finding R and S for Tricky Examples
Key Questions
-
Answer:
Here's how I do it.
Explanation:
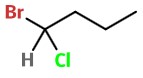
Step 1. Draw the structure of 1-bromo-1-chlorobutane.
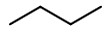
Step 2. To get the stereochemical structure, start with the structure of butane.
Step 3. At
#"C-1"# , draw a wedge, a dash, and a solid bond.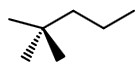
Step 4. Put the
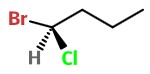
#"H"# on the dashed bond. Then add the#"Br"# and#"Cl"# to the remaining bonds in any order.We have a 50:50 chance of getting it right. Here's one possibility.
Step 5. Assign priorities to the groups.
#"Br"# = 1;#"Cl"# = 2;#"C-2"# = 3;#"H"# = 4.Step 6. Assign stereochemistry
Then
#"Br"# →#"Cl"# →#"C-2"# = 1 → 2 → 3 goes in a counterclockwise direction (#S# ).We got it right!
So this really is (
#S# )-1-bromo-1-chlorobutane.If we had gotten it wrong, we would have interchanged any two of the groups.
For example, in the diagram above, interchanging the
#"Cl"# and#"Br"# atoms converts the (#S# ) isomer to the (#R# ) isomer. -
Answer:
The structure is below...
Explanation:
Both
#Cl# groups should be pointing backward. After assigning priorities, the rotation will be counter-clock wise (which means#S# ) however because the highest priority group is pointing backward, the#S# becomes#R# .
A video on labeling
#R# and#S# is here:
-
Answer:
Because you have to represent a three dimensional structure on two dimensional paper.
Explanation:
This is always problematic, and you can make mistakes even with reference to molecular models. It depends how well your spatial awareness can translate the structure to the printed page. Good luck.