How do you draw lewis structures for polyatomic ions?
1 Answer
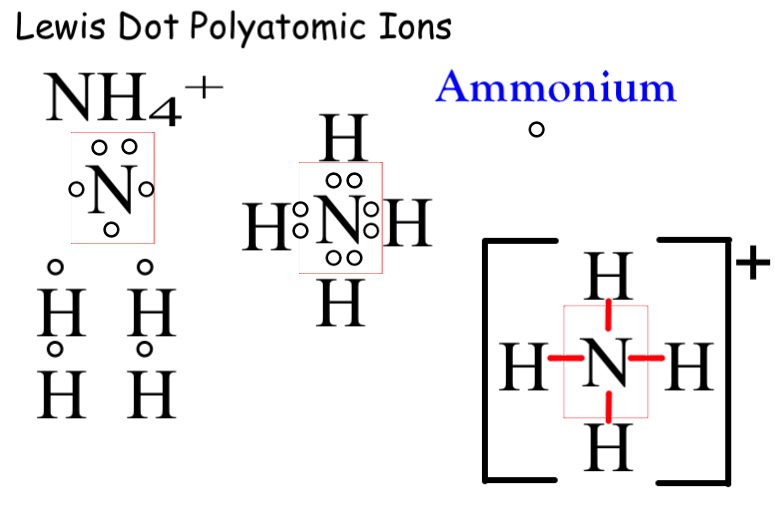
For the positive polyatomic ammonium, we look at the three bonding sites for nitrogen and the four possible bonding sites on the four Hydrogen atoms. Three of the hydrogen bond covalently to the nitrogen but the fourth hydrogen must give up its sole electron and bond to the top pair that remains on nitrogen. This creates electrochemical imbalance of +1. To symbolize this we show single bonds for each hydrogen to the nitrogen in the center. Because it is a polyatomic we place the structure in brackets and place the charge as a superscript outside the bracket.
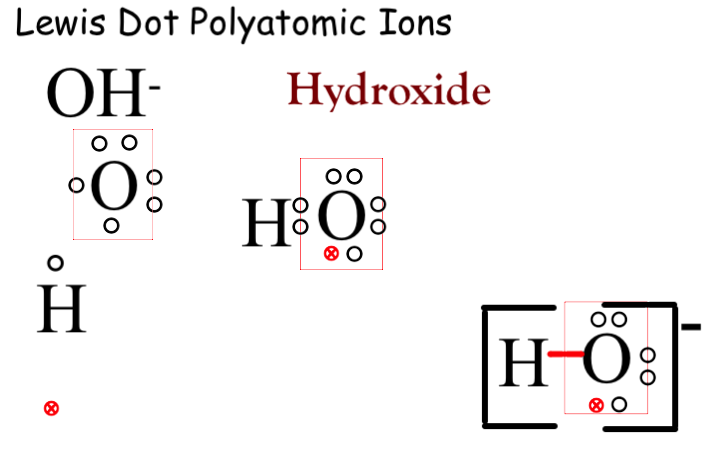
For the negative polyatomic hydroxide the single hydrogen can only bond to one of the bond sites on the oxygen. This leaves an empty bond site which can be filled by an atmospheric electron. This creates an electrochemical imbalance of -1. To symbolize this we show a single bond for the hydrogen to the oxygen. We then add an extra electron to complete the rule of octet for the oxygen atom. (I was always told to identify the atmospheric electron by making the symbol stand out.) Because it is a polyatomic we place the structure in brackets and place the charge as a superscript outside the bracket.
Please watch the video below for more examples and a clear explanation.
SMARTERTEACHER YouTube

