How do you draw VSEPR structures?
1 Answer
Aug 3, 2017
How do you draw a line; how do you draw a triangle; how do you draw a tetrahedron; how do you draw a Platonic solid?
Explanation:
See this link for a start.
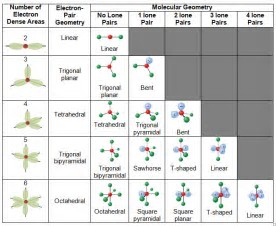
Depending on the number of electron pairs surrounding the central atom, electronic geometry assumes shapes of:
Of course the final molecular geometry depends on whether the electron pairs are lone pairs or bonding pairs. The descent of symmetry from say methane, tetrahedral, to ammonia, trigonal pyramidal is well-known.....but electronic shape/geometry assumes ONE of the 5 given geometries.

