How do you know how many lines to draw in a Lewis structure?
1 Answer
The period an element is on, is in relation to the number of orbitals its Bohr-Rutherford diagram requires.
Explanation:
I believe you're confusing Lewis (dot) diagrams and Bohr-Rutherford diagrams.
Lewis diagrams only depict the valence electrons, while Bohr-Rutherford diagrams depict all of the electrons present in one atom.
In Bohr-Rutherford diagrams, the period an element is on, is in relation to the number of orbitals its the diagram requires.
For example, phosphorus.
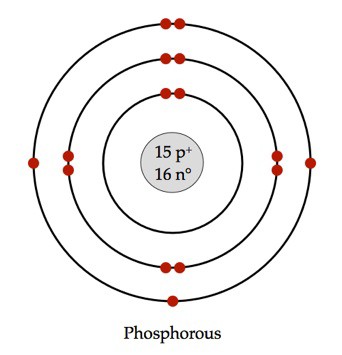
Phosphorus is on the 3rd period, meaning there will be 3 orbitals around the nucleus.
As you go down a period, the number of electrons allowed on an orbital increases.
A Lewis dot diagram will have 5 valence electrons, because it's in the 5th group.

The position of the valence electron pair is unimportant in this case
When drawing the Lewis structure of a compound, you may use lines to represent an electron pair.
Hope this helps :)

