How do you write/draw a Lewis structure for #N#?
1 Answer
Apr 11, 2016
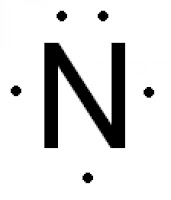
Explanation:
The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen.
The electronic configuration of Nitrogen is 1
The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.
Nitrogen atom has 5 valence electrons, so its Lewis dot symbol for N is
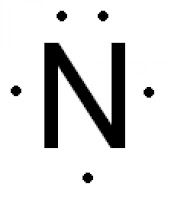
This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has.
Video from: Noel Pauller
Hope this helps!


