How does a limiting reagent affect the products in a chemical reaction?
1 Answer
The presence of a limiting reagent will reduce the amount of products a particular reaction can form.
The reactant that acts as a limiting reagent will be consumed first by the reaction, in essence leaving the other reactant(s) in excess. This implies that the amounts of products the reaction forms will depend on the limiting reagent.
Here's an example of why that happens. The reaction between hydrogen gas and nitrogen produces ammonia according to the balanced chemical equation
Notice that 1 mole of
Now take a look at the following image.
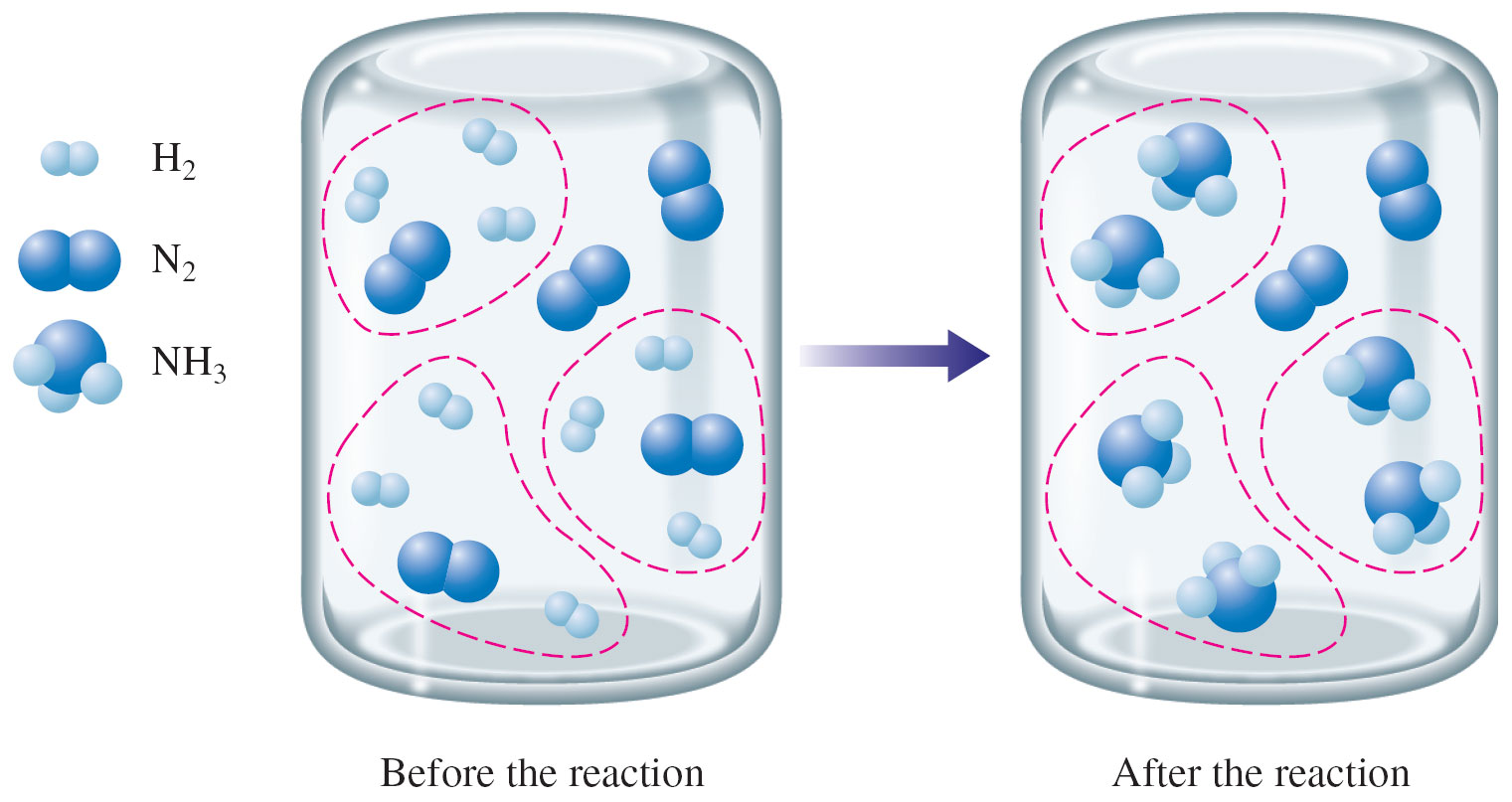
Notice that the left container has 9 molecules of
In order for all of the nitrogen molecules to react, you'd need
Since you only have 9 molecules of
As you can see, the fact that not all of the nitrogen reacted influenced how many ammonia molecules were produced. Instead of 5 molecules, this reaction will only produce 3 molecules of ammonia.

