How does a terminal alkyne react in a Grignard reaction, will the carbonyl carbon attack the terminal carbon?
1 Answer
Jan 21, 2016
Not in a nucleophilic attack, no.
Actually, the pKa of acetylene for example, is
Remember, a Grignard reagent is basically one of the strongest nucleophiles out there, but it is also one of the strongest bases out there. It's essentially an anionic alkane.
It's much more likely that it will steal a terminal proton from the alkyne like so:
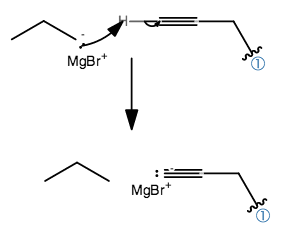
And that deactivates the Grignard reagent. Whoops! The acetylide can still be a pretty nice nucleophile, but not quite as great. The

