How does acid rain change the conditions in small lakes, streams, oceans, rivers?
1 Answer
By acidification
Explanation:
When carbon dioxide dissolves into seawater, a portion of it creates carbonic acid,
The natural pH of the seas is approximately 8.1, which means it is somewhat alkaline (not acidic). The reactions that take place due to more carbon dioxide in the atmosphere (summarized above) release hydrogen ions, which make the water less alkaline.
Compared to preindustrial times, the seas have already experienced a drop in pH of about 0.1 and it is highly likely that marine pH will fall by another 0.3 by the end of this century.
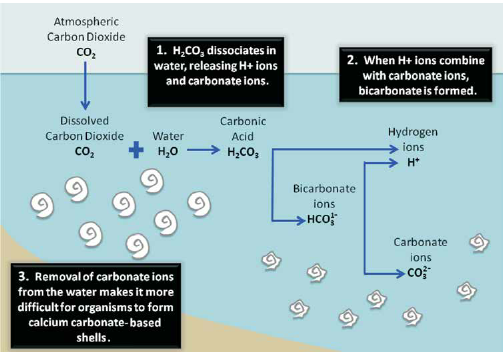
If that happens, the seas will be more acidic than they have been for hundreds of millions of years. So, this will cause end of some creatures and environmental conditions.
For instance, carbonate is needed to build the calcium carbonate in shells and other body parts of marine creatures such as snails, starfish, clams, urchins and others. Read more here.
Some of the most important organisms likely to be affected by a lack of carbonate due to atmospheric
Similar to the seas, rivers, lakes, and streams will suffer from increasing
References: Masters, G.M. and Ela, W. P. (2008). Introduction to Environmental Engineering and Science (3rd edition). Pearson International Education, Upper Saddle River, NJ, USA.
To learn more about ocean acidification, see this link.


