In electrophilic aromatic substitution, an electrophile #"E"^+# first attacks the π system of the benzene ring, and then a Lewis base in the reaction mixture removes the #"H"# atom to regenerate the aromatic π system.
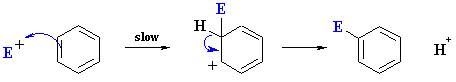
Here are some typical electrophilic aromatic substitution reactions.
#bb"Reaction Type"color(white)(XXXX)bb"Typical Equation"color(white)(XXXXXll)bb"E"^+#
#"Halogenation"color(white)(XXl)"C"_6"H"_6 + "Cl"_2 stackrelcolor(blue)("FeCl"_3, Δ)(→)"C"_6"H"_5"Cl"color(white)(XXXXl)"Cl"^+#
#"Nitration"color(white)(XXXXl)"C"_6"H"_6 + "HNO"_3 stackrelcolor(blue)("H"_2"SO"_4, Δ)(→)"C"_6"H"_5"NO"_2color(white)(Xll)"NO"_2^+#
#"Sulfonation"color(white)(XXXl)"C"_6"H"_6 + "SO"_3 stackrelcolor(blue)("H"_2"SO"_4, Δ)(→)"C"_6"H"_5"SO"_3"H" color(white)(XX)"SO"_3"H"^+#
#"FC alkylation"color(white)(XXl)"C"_6"H"_6 + "RCl" stackrelcolor(blue)("AlCl"_3, Δ)(→)"C"_6"H"_5"Cl" color(white)(XXXX)"R"^+#
#"FC acylation"color(white)(XXll)"C"_6"H"_6 + "RCOCl" stackrelcolor(blue)("AlCl"_3, Δ)(→)"C"_6"H"_5"COR" color(white)(X) "RCO"^+#

