How does carbon dioxide change the acid-base condition of a solution?
1 Answer
The presence of carbon dioxide will increase a solution's acidity because of the formation of carbonic acid.
When carbon dioxide dissolves in aqueous solution, it reacts with water to form carbonic acid,
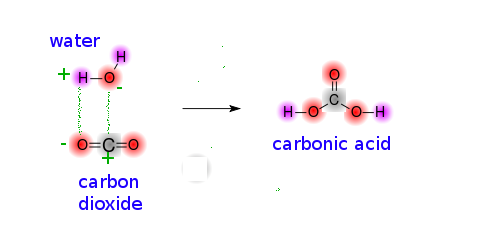
and
The carbonate acid will then release either one or both of its protons, producing bicarbonate,
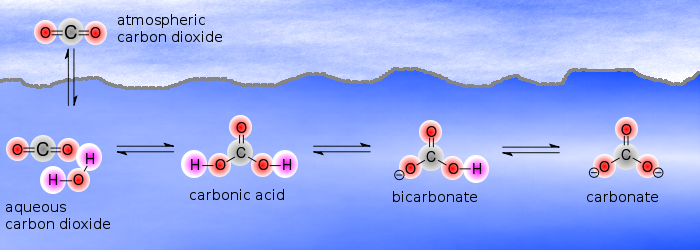
This is essentially the process responsible for ocean acidification.
Read more on that here:

