...the Lewis structure I would write is....
#""^(-):C-=O:^(+)#
...i.e. the HOT end of the ligand is the carbon, i.e. the donor with negative charge....and this typically binds to a metal centre in a Lewis base, Lewis acid interaction.....#Mlarr""^(-):C-=O:^(+)#.
However, carbon monoxide is often found coordinated to LOW valent transition metal centres, i.e. metals in low oxidation states....i.e. #Cr^0#, #Ni^0#...we invoke some mechanism by which the metal can back-donate electron density to the ligand, and this is what we mean we speak of the #pi-"acidity"# of carbon monoxide, it accepts electrons as well as donating them.... It engages in #sigma-"bond"# donation, but it also engages in #pi# back-donation, because the #pi-"antibonding orbitals"# of carbon monoxide are (i) empty, and (ii), the right geometry, and (iii) the right energy to receive electron density from the FILLED #"d-orbitals"# of the metal centre....
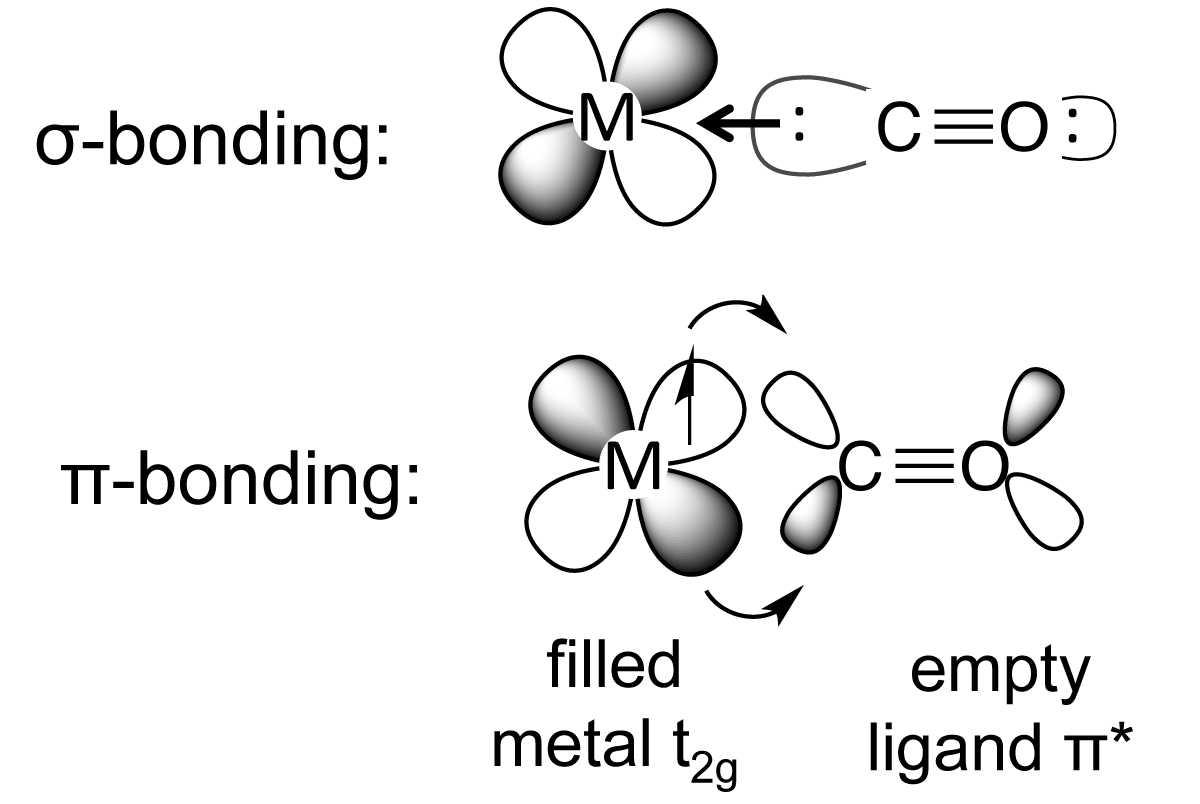
The bonding interaction is positive overall...i.e. the metal carbon bond is strengthened....but because electron density is DONATED to an empty antibonding orbital of the #C-=O#, with respect to carbon monoxide, the #C-O# bonding interaction is diminished, and the physical manifestation of this is the decrease in the #nu_(CO)# stretching frequency in the IR spectrum....


