How does collision theory relate to reaction rates?
1 Answer
Collision theory states that chemical reactions occur between particles when the molecules collide together, if they have enough energy to cause a reaction.
Explanation:
So in theory, we could increase the rate of reaction if we have more collisions, or if more collisions have enough energy.
This is known as the activation energy - the energy that particles must collide with for a chemical reaction to occur. Not all collisions result in a reaction - only those with enough energy.
With this knowledge, we have 3 ways of increasing the rate of reaction:
1) Increase the temperature. If the temperature is higher, particles have a higher kinetic energy so will be moving faster. This means that more collisions will occur, so the rate of reaction increases. Additionally, the particles themselves have more energy, so more collisions are likely to be above the activatioin energy
2) Increasing the pressure of concentration. This means there are more particles per unit volume in an area, so particles will be colliding more so there will be more frequent successful collisions
3) Increasing the surface area.
Consider the image below, of aluminium ribbon on the left and aluminium powder on the right. Which will react faster?
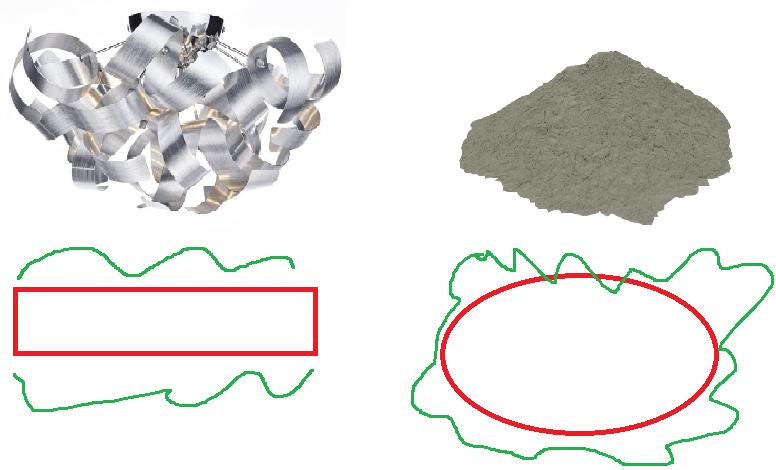
The aluminium ribbon has a much smaller surface area- it only has the top and bottom faces of the ribbon - while the powder has all the area of the powder. This larger area means that there is more area where the particles can collide, leading to more frequent successful collisions.
The other way of increasing the rate of a reaction is to use a catalyst. This provides an alternative reaction pathway, where the activation energy is lower. This means that there are more collisions above the activation energy so the reaction occurs faster. Note, a catalyst is not used up during a reaction so can be reused - this lowers the cost in industrial processes, especially as lower temperatures and pressures can be used.
