How does competitive enzyme inhibition affect a chemical reaction?
1 Answer
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity.
The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible.
Competitive inhibition: substrate (S) and inhibitor (I) compete for the active site.
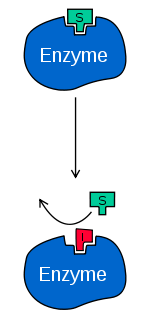
You can see that the substrate can not fit into the active site if the inhibitor already is there. So it is blocked. The reaction will stop at this point.
Enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell.
In competitive inhibition, the substrate and inhibitor cannot bind to the enzyme at the same time. This usually results from the inhibitor having an affinity for the active site of an enzyme where the substrate also binds.
Natural poisons are often enzyme inhibitors that have evolved to defend a plant or animal against predators. These natural toxins include some of the most poisonous compounds known.
Other artificial enzyme inhibitors block acetylcholinesterase, an enzyme which breaks down acetylcholine, and are used as nerve agents in chemical warfare.
An example of a medicinal enzyme inhibitor is sildenafil (Viagra), a common treatment for male erectile dysfunction.
Drugs also are used to inhibit enzymes needed for the survival of pathogens. For example, bacteria are surrounded by a thick cell wall made of a net-like polymer called peptidoglycan.
Many antibiotics such as penicillin and vancomycin inhibit the enzymes that produce and then cross-link the strands of this polymer together. This causes the cell wall to lose strength and the bacteria to burst.

