How does electronegativity affect a chemical bond?
1 Answer
Apr 5, 2018
As a general rule of thumb, if the difference between the electronegativities of two elements is greater than 1.7, then the compound formed will be an ionic compound.
Explanation:
Electronegativity is a numerical representation of the energy required of any element to form bonds with other elements.
I've attached a table for you:
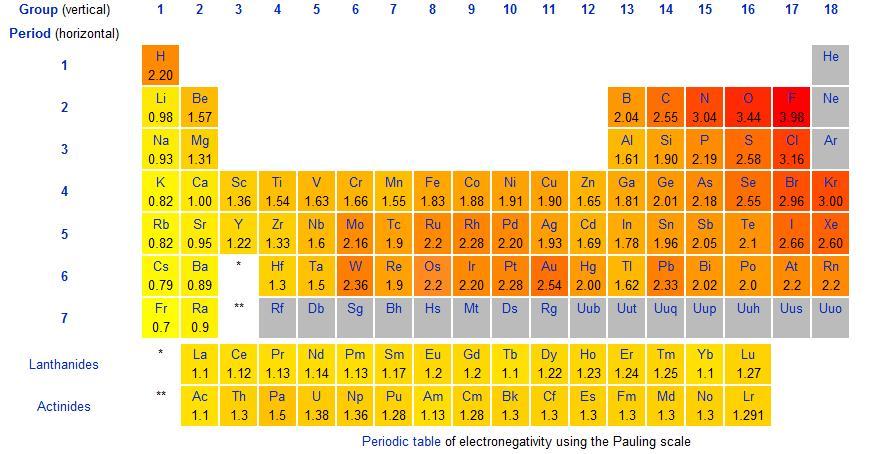
If the difference between two elements (and one is a metal while the other is a non-metal) is above 1.7, then the compound formed will be an ionic compound and an ionic bond is formed.
If the difference between two elements is smaller than 1.7, then the compound formed will be a covalent compound. Hope this helps!