How does electronegativity change across a period?
1 Answer
Would it not increase due to increasing
Explanation:
As always, electronegativity, the tendency of an atom involved in a chemical bond to polarize electron density towards itself, is a function of (i) nuclear charge, and (ii) shielding by other electrons. Incomplete electronic shells shield the nuclear charge VERY ineffectively with the result that electronegativity increases ACROSS the Period from LEFT to RIGHT as we face the Table, but DECREASES down the Group.
And as chemists, as scientists, we should look at some data...even tho electronegativity is a bit of a contrived measurement...the scales have been normalized to give values between
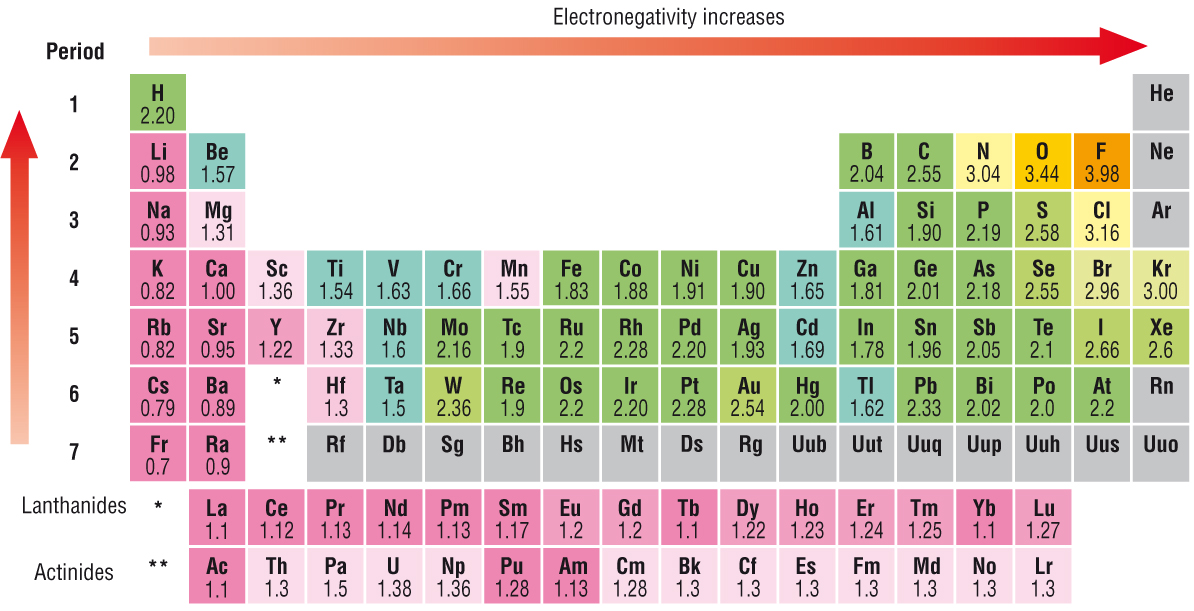
Are these values roughly consistent with what we have argued? The electronegativity of fluorine at

