How does light exhibit traits of a particle?
1 Answer
Light's particle-like traits are best explained by the photoelectric effect, the theory that Albert Einstein won his Nobel Prize for.
The photoelectric effect reffers to the emission (or ejection) of electrons from the surface of a metal in response to incident light. Energy contained by the incident light is absorbed by the electrons in the metal; this gives the electrons enough energy to be emmitted from the surface of the metal.
An interesting thing was dicovered when trying to apply Maxwel's wave theory of light to this experiment. According to the classical wave approach. the energy of the electrons should increase with the intensity of the incident light - the more intense the light, the bigger the average energy carried by an emmitted electron.
However, experimental data showed that the energies of the emmitted electrons were independent of the intensity of the light; these energies were proportional to the frequencies of the incident light.
Here is where Einstein's theory provided an explanaition. Einstein argued that this could be explained if light was composed of "bundles", or photons; when a photon strikes the metal's surface, its energy was transferred to the electron - much like when two billiard balls collide. This was the ultimate evidence for light's particle nature.
The energy carried by a photon is mathematically described as
Out of this theory the quantum theory of light was formulated - the idea that light exists as tiny particles called photons, which is part of the dual nature of light theory.
Here's a diagram showing the photoelectric effect for potassium:
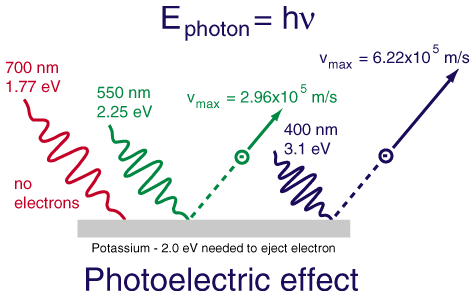
I HIGHLY recommend visiting this website and checking out a simulated version of the photoelectric effect, it's great:
http://phet.colorado.edu/en/simulation/photoelectric


