How does shielding effect affect ionization energy?
1 Answer
It reduces ionization energy
Explanation:
The shielding (or screening) effect is like a barrier effect. Look closely at the following image.
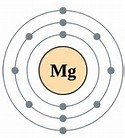 bing images
bing images
It's a Magnesium atom with 3 energy levels (shells), two of which are filled. Energy levels contain electrons and all electrons are negatively charged. Since they have the same charge, they will repel each other. The outer electrons are being shielded from the full attractive force of the nucleus by the two electron filled energy levels before them.
The more filled inner shells that an atom has, the more the outer electrons will be shielded from the nucleus' attraction. This is because there are more electons to repel them. So, in the case of Magnesium, 10 electrons are screening a portion of attractive force from the two valent electrons.
This is why the first ionization energy decreases down a group. Each subsequent element in the group will be gaining a shell, so the electrons on the valent shell will be blocked out from increasing amounts of attractive force by more and more electrons.
And of course, not as much energy will be needed to remove these electrons since they are already loosely held because of the repulsion of inner electrons.
Electron shielding has a negligible effect on electrons in the same energy level.

