How does the arrangement of the particles in a liquid compare to that of a gas?
1 Answer
Jun 7, 2016
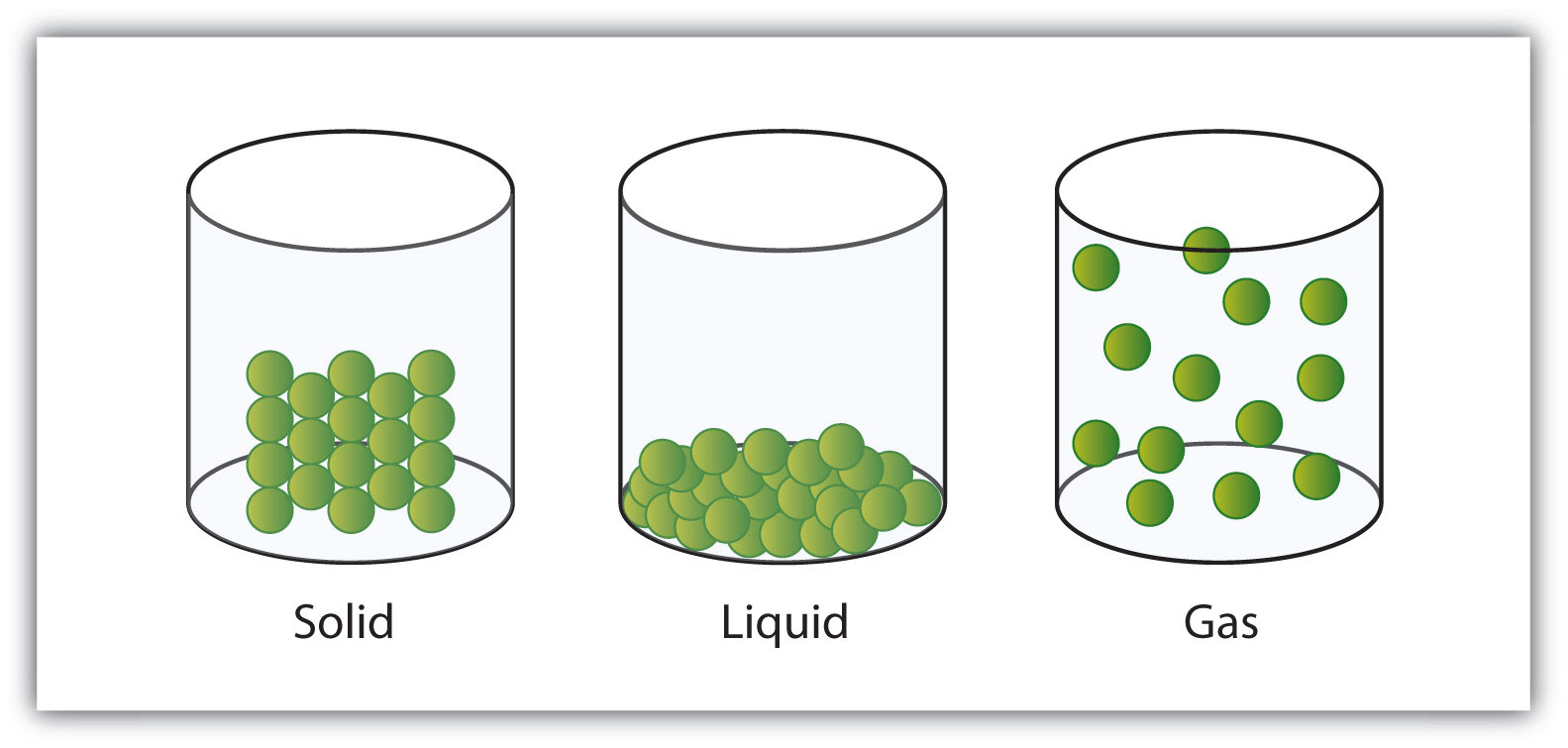
Molecules in a gas are far apart as compared to liquid. Liquid molecules are closely held with molecular forces which are weaker than that of solids.
Explanation:
Particles in a solid are tightly packed and are not able to move because of high intermolecular attraction and less intermolecular spaces. Liquids have greater intermolecular spaces and less intermolecular attraction and the particles are free to move, but they do have fixed volume. Gases, on the other hand, do not have fixed volume or shape. They have large intermolecular spaces and very weak intermolecular attractions. hence they are very loosely packed.