How does the collision theory explain reaction times?
1 Answer
The poorer the participants are at colliding, the fewer the collisions, and the slower the reaction.
When we model reactions, it is convenient to do so with a reaction coordinate diagram, which shows the state of the reaction based on the current relative energy of the system.
In a basic reaction with no intermediates, we have:
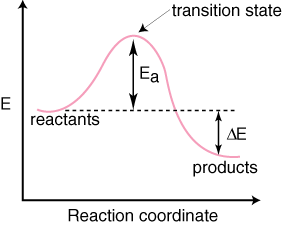
The hump in the diagram indicates an activation barrier; it's like an energy threshold.
A transfer of energy due to collisions causes an energy buildup, especially in a closed system, facilitating the overcoming of the energy barrier. Afterwards, the reaction can go to products.
The basic tenets of collision theory are that:
-
Molecules have to collide for a reaction to occur
This is the basic requirement; no collision, no reaction.
-
They have to collide at the proper orientation in the right way
This is further required for a reaction to occur. Some collisions don't affect the target molecule in the correct way, and nothing sufficient happens afterwards.
-
They have to have enough energy to overcome the activation barrier.
If this doesn't happen, the reaction cannot proceed past the transition state and it will never finish.
Some reactions have frequently effective collisions, whether it's because of a high temperature, or inherently high molecular speeds (such as in gases), or whatever other reason, and go to products quickly because the energy transfer is effective.
Some others have infrequently effective or frequently ineffective collisions, and the reactions go to products slowly because the energy transfer is ineffective.
When a reaction is kinetically unfavorable (
The poorer the participants are at colliding, the fewer the collisions, and the slower the reaction.

