How is matter classified?
1 Answer
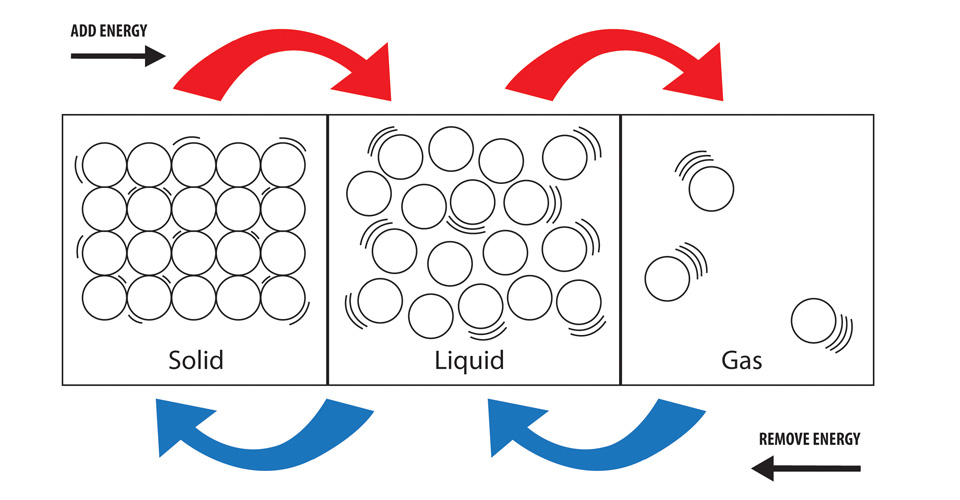
There are three stages of matter, solid, liquid, and gas.
Solid:
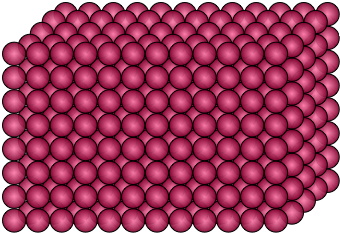
Particles in a solid are very closely packed, with seemingly no room to move around. This is why solids are hard and rigid. The molecules within a solid are so densely packed, they have no room to move or bend, therefore they hold their shape. Solids will not take the shape of their container, and have a definite shape and volume.
Liquid:
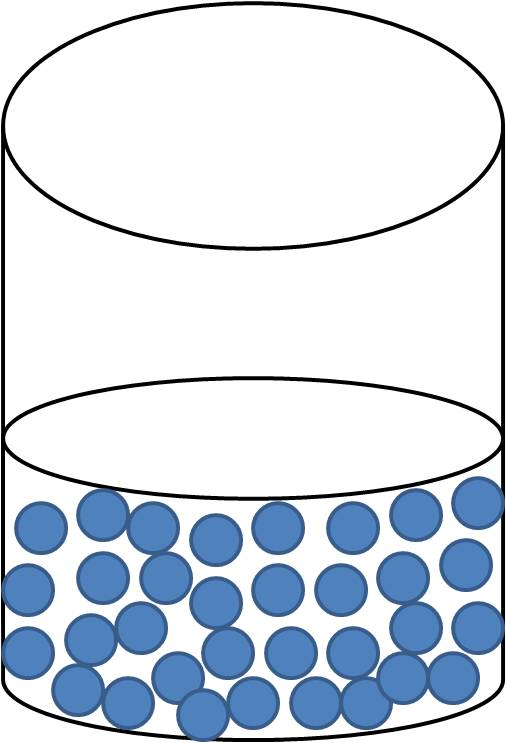
Particles within a liquid possess room to move around, but not enough energy to become a gas. Molecules in a liquid are able to slide and move past each other which allows the liquid to change shape. Loosely packed particles in a liquid allow the liquid to change to take the shape of their container. Liquids will change shape, and have a definite volume.
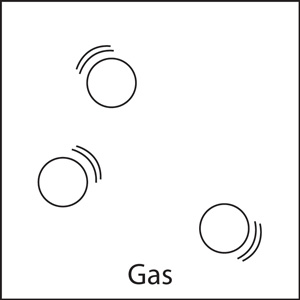
Gas:
Particles in a gas are relatively far away from each other. Their only boundary is when the particles either bounce off each other or their container. Gasses will completely fill their container and have no definite shape or volume.