How is molecular weight related to intermolecular forces?
1 Answer
Jan 8, 2018
Well, the heavier the molecule, the more electrons it contains.....
Explanation:
...and the more electrons it contains, the LARGER, and the MORE POLARIZABLE the electron cloud of the molecule is, and thus the GREATER the possibility of intermolecular interaction by dispersion forces.
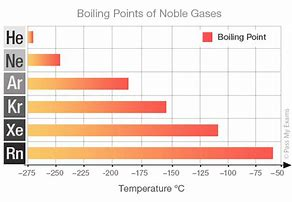
This trend is nicely illustrated by the Noble Gases. the which are all room temperature gases, with minimal forces of intermolecular (i.e. here interatomic) interaction.....