How many constitutional isomers are possible for the formula C4H9Cl?
1 Answer
Jan 1, 2016
There are four possible constitutional isomers of
Explanation:
First draw all the constitutional isomers of
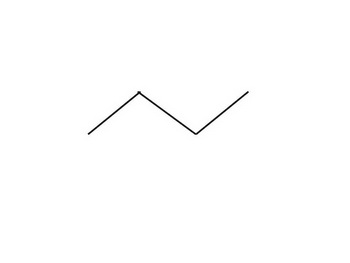
(from hardinars.tk)
and
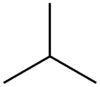
Then put a
From the straight-chain alkane, we get
1-Chlorobutane

and
2-Chlorobutane

From the branched-chain alkane, we get
1-Chloro-2-methylpropane

and
2-Chloro-2-methylpropane

And there are your four constitutional isomers of

