How many electrons can have the quantum number set n=3 and ml=-2?
1 Answer
Two electrons.
Explanation:
As you know, four quantum numbers are used to describe the position and spin of an electron that's part of a given atom, with each electron being described by an unique set of quantum numbers.
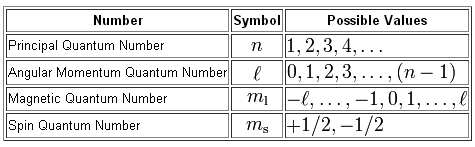
In your case, the problem provides with the values of the principal quantum number,
More specifically, the problem provides you with the energy level on which the electron resides, and the specific orbital in which it is located.
Your job now will be to determine how many subshell can hold that specific orbital.
Notice that the value of the magnetic quantum number depends on the value of the angular momentum quantum number,
In order to have
Since you need to have
#l = 2 -># the d-subshell
Since each orbital can hold a maximum of two electrons, the number of electrons that can share the two quantum number
#n = 3, l=2, m_l = -2, m_s = -1/2#
This set describes an electron located on the third energy level, in the 3d-subshell, in the
#n = 3, l = 2, m_l = -2, m_s = +1/2#
This set describes an electron located on the third energy level, in the 3d-subshell, in the

