How many electrons does boron have?
2 Answers
Boron atomic number 5 has five electrons in its ground state.
Commonly Boron will lose 3 electrons leaving 2 electrons in its most common ionic form.
Explanation:
The atomic number gives the number of protons. Protons which have a positive charge are balanced by an equal number of electrons in a neutral atom.
Boron number 5 has five protons and therefore as a neutral atom also has five electrons.
Boron has an electron configuration of
The most stable electron configuration for Boron is
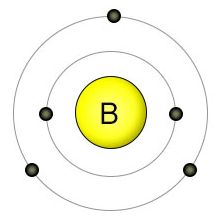
Boron has five electrons.
Explanation:
Boron is element number 5 in the Periodic Table.

That means it contains five protons and five electrons.
They are arranged with two electrons in the first energy shell and three in the second.
A chemist would say that the electron configuration of boron is

