How many electrons in an atom can have #n + l =6# ?
The answer is 18. How do you get this answer?? Thanks!
The answer is 18. How do you get this answer?? Thanks!
1 Answer
Explanation:
As you know, we use four quantum numbers to describe the location and spin of an electron in an atom.
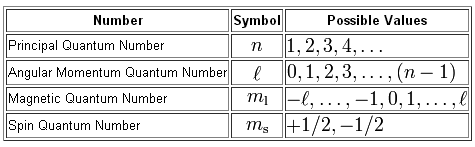
In your case, you must find the number of electrons that can have
#n + l = 6" " " "color(orange)("(*)")#
The angular momentum quantum number,
#l <= n-1#
Right from the start, you can tell that the first energy level that can hold electrons that satisfy condition
#n = 3 implies l = (0, 1, 2}#
you can't have a pair that matches the given condition
#3 + 0 < 6" "color(red)(xx)#
#3 + 1 < 6" "color(red)(xx)#
#3 + 2 < 6 " "color(red)(xx)#
The same is true, of course, for
Now, for
#n = 4 implies l = {0, 1, 2, 3}#
#4 + 2 = 6" "color(green)(sqrt())#
For the angular momentum quantum number, you have
#l=0 -># the s-subshell#l=1 -># the p-subshell#l=2 -># the d-subshell
The d-subshell holds a total of
#m_l = {-2, -1, 0, 1, 2} -># for the d-subshell
Now, each orbital can hold a maximum of two electrons, which means that a total of
#5 color(red)(cancel(color(black)("d-orbitals"))) * "2 e"^(-1)/(1color(red)(cancel(color(black)("orbital")))) = "10 e"^(-)#
can have
#n=5 implies l = {0, 1, 2, 3, 4}#
This time, you can have
#5 + 1 = 6" "color(green)(sqrt())#
The p-subshell holds a total of
#m_l = {-1,0,1} -># for the p-subshell
Each of those orbitals can hold
#3 color(red)(cancel(color(black)("p-orbitals"))) * "2 e"^(-)/(1color(red)(cancel(color(black)("orbital")))) = "6 e"^(-)#
can have
#n=6 implies l = {0,1,2,3,4,5}#
This time, you have
#6 + 0 = 6" "color(green)(sqrt())#
The s-subshell holds a single orbital, since
#m_l = 0 -># for the s-subshell
This means that only
#1 color(red)(cancel(color(black)("s-orbital"))) * "2 e"^(-)/(1color(red)(cancel(color(black)("orbital")))) = "2 e"^(-)#
can have
Therefore, the total number of electrons that can have
#overbrace("10 e"^(-))^(color(blue)(4 + 2 = 6)) + overbrace("6 e"^(-))^(color(darkgreen)(5 + 1 = 6)) + overbrace("2 e"^(-))^(color(purple)(6 + 0 = 6)) = "18 e"^(-)#

