How many isomers of C_4H_8Cl_2C4H8Cl2 are here? How do you tell if one structure is an isomer of another?
1 Answer
Jun 28, 2016
I count 13 isomers of
Explanation:
I find that the easiest way to tell if one structure is an isomer of another is to write their IUPAC names.
Each isomer will have a unique name.
We use a systematic procedure to generate the structures.
A. Base chain is 1-chlorobutane
1,1-Dichlorobutane
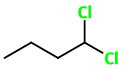
(

(

1,4-Dichlorobutane
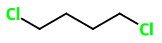
B. Base chain is 2-chlorobutane
2,2-Dichlorobutane
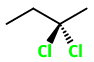
(
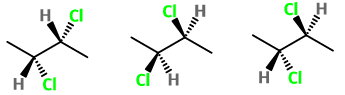
C. Base is 1-chloro-2-methylpropane
1,1-dichloro-2-methylpropane
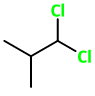
1,2-Dichloro-2-methylpropane
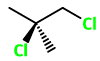
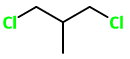
1,3-Dichloro-2-methylpropane