How many lone pairs of electrons are on the #Xe# atom in #XeF_6#? A) 0 B) 3 C) 2 D) 1
1 Answer
May 25, 2018
D)
Explanation:
First, let's draw the Lewis Structure for
- The total number of electrons in
#XeF_6# is#8+(7xx6)=50# electrons. #Xe# will be the central atom. So, let's just connect#Xe# and#F# atoms with a single bond each.
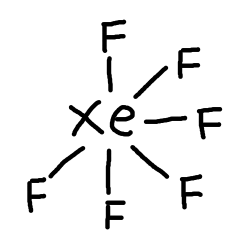
- We have
#50-12=38# electrons left to draw on.
This means that we have enough electrons to not need double bonds and complete the octet for#F# using lone pairs.
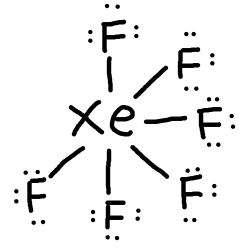
- However, we still have
#38-36=2# electrons left to use.
These electrons will need to be placed on#Xe# , since#Xe# , having its valence electrons in the#5p# orbital, have empty#5d# ,#4f# , and#5f# orbitals—allowing it have more electrons than an octet.
(For more information on exceeding octets, check out this article on Wikibooks!)
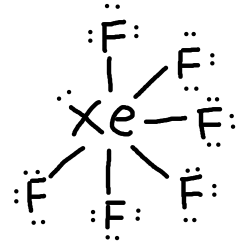
So, there are

