How many resonance structures are there for NO2?
1 Answer
Aug 4, 2014
There are two major resonance structures for
Explanation:
You must first draw the Lewis structure for
You find that you can draw two Lewis structures.
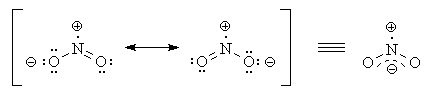
However, there are two even better Lewis structures.
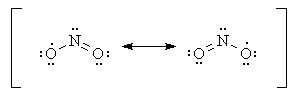
The
Every atom has a formal charge of zero.
These are the two major resonance contributors to

