How many sigma and pi bonds are present in buta-1-3-di-ene ?
2 Answers
May 12, 2018
Consider 1,3-butadiene,
 puu.sh
puu.sh
Each double bond has one
Moreover, each
Hence, this species has,
2
9
May 12, 2018
Explanation:
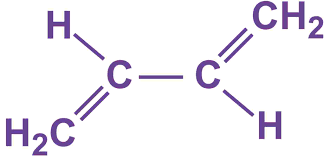 http://www.essentialchemicalindustry.org/chemicals/buta-13-diene.html
http://www.essentialchemicalindustry.org/chemicals/buta-13-diene.html
As you can see, there are only two double bonds, which result in two



