How many triple bonds are present in the compound #C_2H_2#?
1 Answer
One.
Explanation:
Your best strategy when dealing with such questions is to draw the Lewis structure of the molecule.
So, acetylene,
Now, when you draw a Lewis structure, you need to make sure that the atoms that are a part of the molecule have a complete octet.
SInce the hydrogen atoms can only form one bond, you know for a fact that will each be bonded to a carbon atom. This will account for
This means that the two carbo natoms must be bonded to each other. In order for them to have completel octets, you need to form a triple bond, which will use up the remaining
The Lewis structure will thus look like this
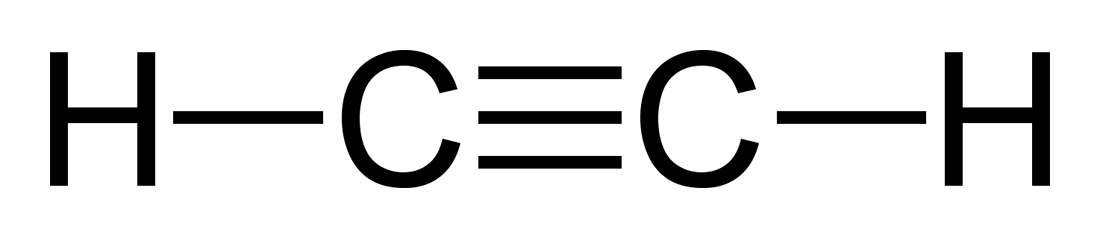
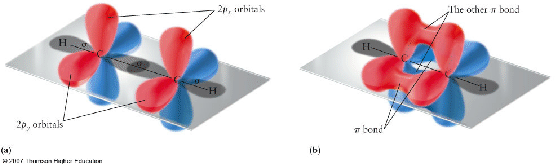
So, now you're redy to answer the question - each acetylene molecule has one triple bond. This triple bond consists of one sigma bond and two pi bonds.